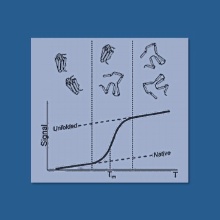
Protein stability is a key factor in successful structural and biochemical research. However, the approaches for systematic comparison of protein stability are limited by sample consumption or compatibility with sample buffer components. Here we describe how miniaturized measurement of intrinsic tryptophan fluorescence (NanoDSF assay) in combination with a simplified description of protein unfolding can be used to interrogate the stability of a protein sample. We demonstrate that improved protein stability measures, such as apparent Gibbs free energy of unfolding, rather than melting temperature T m, should be used to rank the results of thermostability screens. The assay is compatible with protein samples of any composition, including protein complexes and membrane proteins. Our data analysis software, MoltenProt, provides an easy and robust way to perform characterization of multiple samples. Potential applications of MoltenProt and NanoDSF include buffer and construct optimization for X‐ray crystallography and cryo‐electron microscopy, screening for small‐molecule binding partners and comparison of effects of point mutations.
For reference, see In depth interrogation of protein thermal unfolding data with MoltenProt. Kotov, V., Mlynek, G., Vesper, O., Pletzer, M., Wald, J., Duarte, C., Celia, H., Garcia-Ali, M., Nussberger, S., Buchanon, S., Morais-Cabral, J., Loew, C, Djinovic-Carugo, K. & Marlovits, T. Protein Science, 1-17, doi: 10.1002/pro.3986. (2020)