We advise and support with our expertise in the planning and implementation of diverse molecular biology techniques ranging from DNA & RNA work, cloning strategies, analysis of protein-protein interactions to protein expression in E. coli, yeasts and plant systems.
Feel free to contact us if you need support for new projects, if you would like to share devices, or need advice on molecular biology issues.
Cooperation partners:
- Department of Biobased Materials
- Department of Biophysics
- Department of Intelligent Biointegrative Systems
- Department of Plant Biotechnology
- Resaerch Unit Molecular and Synthetic Plant Virology
- Institute of Cell Biology and Immunology
- Institute of Interfacial Process engineering and Plasma Technology, Chemical-Physical Interfaces
Overview of professional expertise in the Molecular Biology Support Unit:

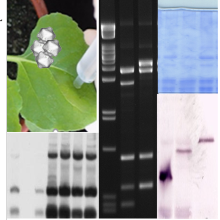
Examples (from left to right): Plasmid map of a recombination-based cloning (Gateway), ethidium bromide-stained DNA restriction fragments separated in agarose gel, detection of viral DNA topoisomers by Southern blot analysis after previous separation in chloroquine-containing agarose gel, analysis of post-transcriptional gene silencing: RNA fraction smaller than 200 nt separated in a polyacrylamide gel stained with SybrGold and associated small interfering (si)RNA Northern blot analysis.
- Various cloning & mutagenesis methods (e.g. PCR techniques, restriction enzyme- & recombination-based cloning, Gibson assembly)
- E. coli work (transformation, plasmid DNA isolation & analysis)
- DNA & RNA isolation from plants, bacteria and yeasts, size fractionation of nucleic acids
- Gel electrophoresis (2D gel electrophoresis, shape and size specific separation techniques, denaturing gels)
- Southern & Northern blot analyses (e.g. plaque lifts, siRNA Northern blot)
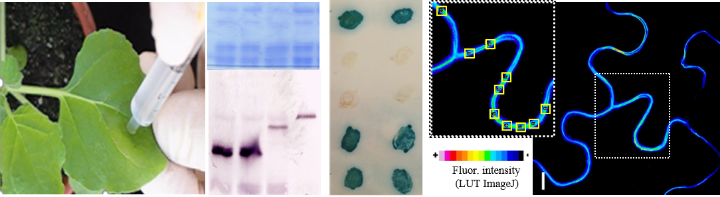
Examples (from left to right): Transient expression of proteins in leaves by the agrobacterial infiltration technique, separation of proteins in an SDS-polyacrylamide gel stained blue with Coomassie and associated Western blot analysis, detection of a test protein interaction in yeast by two-hybrid system and in living plant cells by fluorescence resonance energy transfer (FRET).
- Expression & purification (bacteria, yeasts, plants)
- In vivo labeling
- In vitro & in vivo interaction assay (pull-down assay, yeast two-hybrid system, fluorescence-based: bimolecular fluorescence complementation (BiFC) & fluorescence resonance energy transfer (FRET).
- Enzymatic assay (kinase, methylase)
- Gel electrophoresis (SDS PAGE, Blue Native) & Western blot analysis
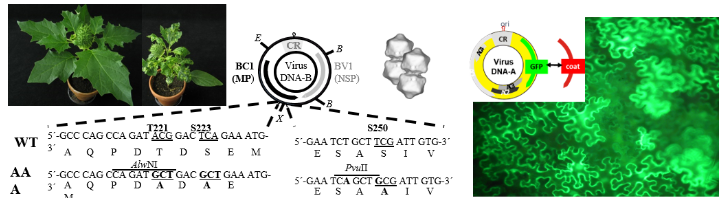
Examples (from left to right): Healthy and geminivirus-infected plant with symptoms, genome map (circular DNA-B) of geminivirus and mutagenesis strategy in BC1 gene, geminivirus particles, genome map geminivirus vector for protein expression in plants here for green fluorescent protein (GFP) and corresponding fluorescence microscopy of GFP-expressing plant cells.
- Transient protein expression in different systems (plants, tissue culture, protoplasts) by different techniques (DNA transfection, agrobacteria-mediated; particle bombardment)
- Agrobacterium tumefaciens work (transformation, plasmid DNA isolation & analysis)
- Establishment of transgenic plants (tissue culture, genotyping, crossing)
- Infection methods (physical and biological techniques), symptom bonitur and analysis of virus-infected plants
- Diagnostics of ssDNA viruses & satellites (Phi29-based rolling circle amplification (RCA)-restriction fragment length polymorphism (RFLP) analysis, PCR, Southern blot analysis)
- Phytoviral engineering (virus-based vector systems for expression and gene silencing).
- Fluorescence microscopy (live observation), inhibitor studies
The equipment of the support unit can be used in consultation. For example:
- EdgeX7 Imaging System (Vilber Lourmat)
- Infinite M200 Pro (Tecan)
- Gene Pulser II electroporation system (Biorad)
- Biolistic PDS-1000/He Particle Delivery System (Biorad)
- FastPrep-24 (MP)
- Equipment for gel electrophoresis
- Incubators
- PCR devices
Team
Rebecca Hummel
Technical assistant

Tatjana Kleinow
PD Dr.Privatdozentin
[Photo: T. Kleinow]