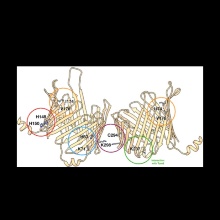
In the past three decades, significant advances have been made in providing the biochemical background of TOM-mediated protein translocation into mitochondria. In the light of recent cryoelectron microscopy-derived structures of TOM isolated from Neurospora crassa and Saccharomyces cerevisiae, the interpretation of biochemical and biophysical studies of TOM-mediated protein transport into mitochondria now rests on a solid basis. In this review, we compare the sub-nanometer structure of N. crassa TOM core complex with that of yeast. Both structures reveal remarkably well-conserved symmetrical dimers of ten membrane protein subunits. The structural data also validate predictions of weakly stable regions in the transmembrane beta-barrel domains of the protein-conducting subunit Tom40, which signal the existence of beta-strands located in interfaces of protein-protein interactions.
For reference, see The structure of the TOM core complex in the mitochondrial outer membrane. Bausewein, T., Naveed, H., Liang, J. & Nussberger, S. Biological Chemistry: doi: 10.1515/hsz-2020-0104 (2020)