Eukaryotes achieve their high performance by distributing their various functions on spatially membrane-bound subcellular compartments. By doing so, however, they are challenged by a severe problem: Subcellular confinement abolishes the principle of free intracellular accessibility for functional gain. Membranes represent a significant diffusion barrier for the majority of molecules in the cell which can only be overcome by a high energy input. Compartmentation or accessibility - an insurmountable contradiction?
To address this question we study the biological physics of how protein polymers thread through nanometer-scale pores. By using quantitative methods of protein biochemistry, cell biology, electrophysiology and single-molecule microscopy and, in collaboration with other research groups, high-resolution cryo-electron microscopy the following topics are addressed:
Protein Translocation
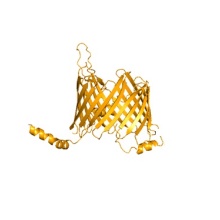
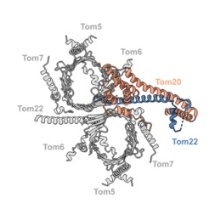
Of particular interest is the elucidation of architecture and function of the protein-conducting mitochondrial membrane-translocase TOM, and the question of how proteins thread through the nanometer-scale pores of this translocase.
Single-Molecule Biosensing
As part of the research iniative Clusters4Future Nanodiag BW of the Federal Ministry of Education and Research (BMBF) and the research network Functional Nanostructures of the Baden-Württemberg Foundation, we are developping new technologies for the integration of biological nanopores into supported lipid bilayers to be used for biosensor applications.
Apoptosis
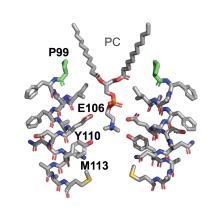
The release of cytotoxic proteins from mitochondria regulated by proteins of the Bcl-2 family is considered a key event of apoptosis. The modification of the Bcl-2 protein BAX with a palmitic acid residue and thus its integration into mitochondrial outer membranes play a crucial role. Based on this observation, we are seeking for new concepts for the control of malignant tumor cells, in which the palmitoylation of BAX is reduced.