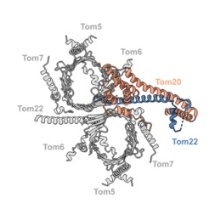
Most mitochondrial proteins are encoded in the cellular nucleus and produced as precursors in the cytoplasm. Precursor proteins are recognized and translocated into the inter-membrane space by the translocase of the outer mitochondrial membrane (TOM). In this work we studied the conformation and composition of the TOM holo complex from Neurospora crassa, including the elusive receptors Tom20 and Tom70. We determined the structure of the complex under native conditions by cryoEM (electron cryomicroscopy), which shows the flexible gatekeeper, Tom20, in two different conformations. Furthermore, we present the structure of the complex with bound preprotein at a late stage of translocation. Overall, we offer insights into the dynamic process of presequence recognition and translocation into mitochondria, dependent on the cooperation of Tom20 and Tom22.
For reference, see Two conformations of the Tom20 preprotein receptor in the TOM holo complex. Ornelas, P., Bausewein, T., Martin, J., Morgner, N., Nussberger, S. & Kühlbrandt, W. Proceedings of the National Academy of Sciences, USA:120 (34), e23014471, doi: 10.1073/pnas.2301447120 (2023)